Phytoplankton Plunder Nutrients
Analysis using models: An understanding the overwhelming importance of biological removal in setting nutrient concentrations in the ocean can be obtained by experimenting with the three different nutrient models. This experiment can be carried out with either the phosphate-only (P), the nitrate-phosphate (NP), or the silicate-phosphate (SiP) models. From the control panel of a model, select 'Model Parameters' and then change the growth rate of all phytoplankton in the model to zero (given that deaths will continue, the phytoplankton populations must then remorselessly decline over time). Click 'Apply and Close' to save the parameter changes and then click 'Run Model' on the main control panel. In the resulting plots you should see a striking effect, that the concentrations of all nutrients in all boxes increase steadily over time. Unlike most runs, they do not converge to an equilibrium value; the lines never flatten out. This is because they have been released from the otherwise incessant removal pressure by phytoplankton (you should also notice a plummeting of phytoplankton numbers at the beginning of the run). With phytoplankton populations no longer able to hold nutrient concentrations down, the nutrients escape to higher values.
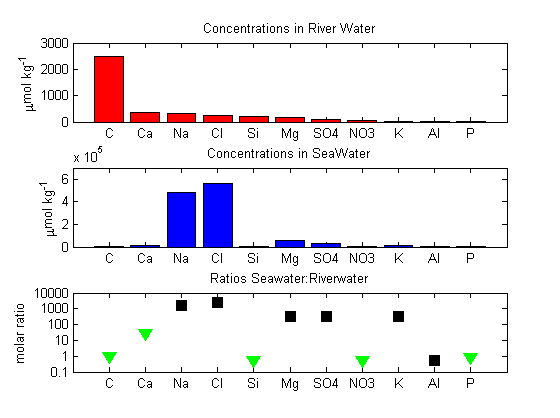
Comparison of dissolved substances in river water and in seawater. The top panel shows concentrations in river water, the middle panel concentrations in seawater, and the bottom panel the ratio of the two (mol/mol, on a log scale). Inverted green triangles denote elements which are widely used by marine organisms, either to make new soft tissues or else to make hard parts (e.g. shells out of calcium carbonate CaCO3 or opal SiO2). Black squares denote elements which are not heavily utilised by living organisms. All biologically-utilised elements are exceptionally scarce compared to expectations based on river supply and comparison to non-utilised elements. Aluminium is also surprisingly scarce, in this case because it tends to stick to falling particles and thereby gets rapidly removed from seawater.
In reality, some of these increases would eventually be capped by another process, such as inorganic precipitation of crystals of solid silica in the case of increasing dissolved silicate. This would start to come into play after dissolved silicate concentrations rise higher than about 1.2 mol kg-1, a lot higher than the normal deep-water concentration of about 0.1 mol kg-1. What happens in the NP and SiP models when only one of the two phytoplankton groups are prevented from growing?
A second way of examining this controlling pressure by phytoplankton is to artificially raise up the nutrient concentrations and then examine the response of the system. This run can be carried out in each nutrient model. First of all make sure to go to 'Model Parameters' from the control panel and then click on 'Default' and 'Apply and Close'. This is in order to remove the previous changes in phytoplankton growth rates. Then go to 'Initial Conditions' and double the deep concentration of nutrients. Save the changes with 'Apply and Close' and then run the model. You should notice a surge in phytoplankton numbers in response to the extra nutrients. This should then result in increased removal of the nutrients in question, leading eventually to a return of both nutrient concentrations and phytoplankton densities to normal values.
Further reading
Tyrrell, T. (2004). Biotic Plunder: Control of the environment by biological exhaustion of resources, In: Scientists Debate Gaia: The Next Century, MIT Press, pages 137-147.