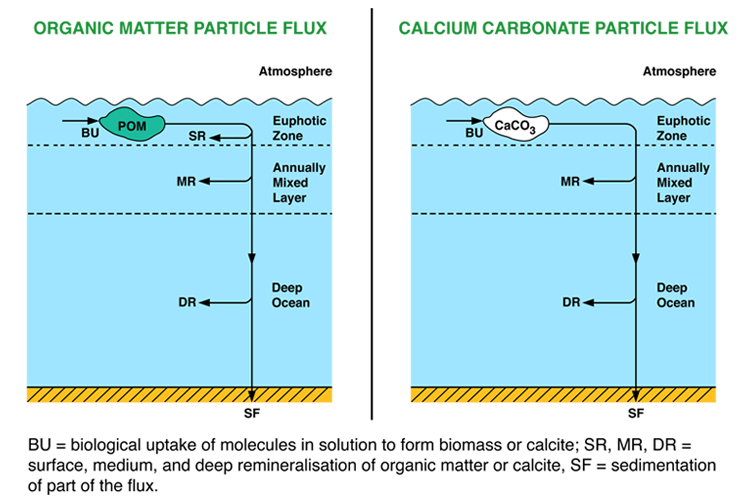
The aim of this carbon model worksheet is to analyse some of the properties of the carbon model. Since the carbon model can be used to address a number of different scientific issues, there is a series of separate exercises for users to attempt.
Worksheets
Worksheet 1: Ocean carbon sink over the next 100 years
Various global change-related processes may alter the behaviour of the ocean carbon cycle. How significant will these human-induced changes be to the ocean’s ability to absorb anthropogenic CO2? What difference will it make to the rise in atmospheric CO2? The model allows you to implement of different scenarios for the future carbon cycle.
Follow this link to access the worksheet.
Worksheet 2: Long term legacy of fossil fuels
While worksheet 1 examines the immediate future, the aim of this worksheet is to examine how much extra CO2 is likely to remain in the atmosphere long after CO2 emissions cease.
Follow this link to access the worksheet.
Worksheet 3: Effect of pH on DIC composition
There are three different species of dissolved inorganic carbon in seawater: dissolved carbon dioxide gas (CO2(aq)), bicarbonate ions (HCO3-) and carbonate ions (CO32-) (actually there is also a fourth, H2CO3, but it is never quantitatively significant). At any one time the total dissolved inorganic carbon (DIC) is made up of some mixture of these three. The relative proportions of these vary as a function of pH.
Follow this link to access the worksheet.
Worksheet 4: Carbonate compensation
Carbonate compensation is the negative feedback process which regulates carbonate ion concentration [CO32-] (or, more strictly, the degree of supersaturation with respect to CaCO3, Ω) in the oceans. When [CO32-] gets too high then more CaCO3 is buried, lowering [CO32-]; conversely when [CO32-] falls too low, less CaCO3 is buried, allowing river delivery to raise [CO32-].
Follow this link to access the worksheet.
Other related pages
References
Chuck, A. et al. (2005). The oceanic response to carbon emissions over the next century: investigation using three ocean carbon cycle models. Tellus B 57, 70-86.
Tyrrell, T. (1999). The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531.
Tyrrell, T. et al. (2007). The long-term legacy of fossil fuels. Tellus B 59, 664-672.
External links
Description of the chemical element carbon
Description of the carbon cycle