Electrochemical sensor development by LEGOS-CNRS
SenseOCEAN partner, LEGOS-CNRS develops miniaturized and autonomous electrochemical sensors to detect silicate and phosphate in sea water.
Because Silicate and Phosphate are non-electroactive species, it is not possible to detect them directly on metallic electrodes. However after complexation with molybdate ions (MoO42-) in acidic conditions, the silico and phospho-molybdic complexes are formed and can be detected by cyclic voltammetry on gold or platinum electrodes.
In order to form these complexes in situ without any addition of liquid reagents, MoO42- and H+ needed can be formed in situ by a simple oxidation of a Molybdenum metal electrode. A Nafion® membrane is also required to isolate the counter electrode from the analytical cell and avoid the reduction of the protons being formed. In this configuration of the cell, acidic conditions are achieved and the silicomolybdic complex is formed and is detected on gold working electrode by cyclic voltammetry.
For the phosphate, another compartment is added in order to avoid the interference of silicate. This extra compartment is connected to the analytical cell with a proton exchange membrane. A first molybdenum electrode is oxidized in this compartment in order to produce enough protons that crossed the proton exchange membrane and are concentrated in the analytical cell. There the second molybdenum electrode is oxidized to produce MoO42-. A ratio MoO42- / H+ of 70 is obtained allowing to complex only the phosphate (not the silicate). See figure below.
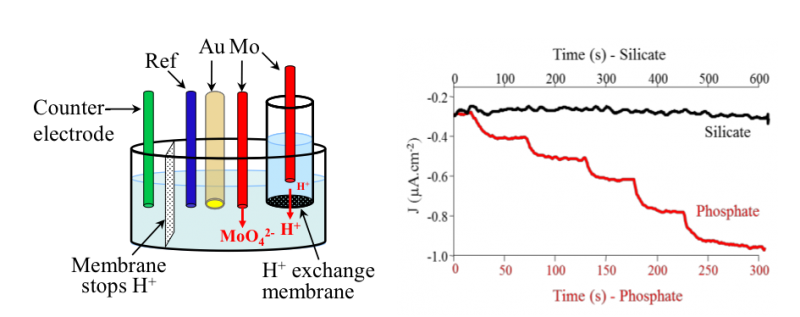
Electrochemical open cell to detect phosphate in seawater without Silicate interference (left) – Evolution of chronoamperometric signal at E=0.29 V obtained with rotating disk electrode (2000 rpm) with standard additions of phosphate and silicate (right)
See later articles for more information on the development of the phosphate and silicate sensors.













